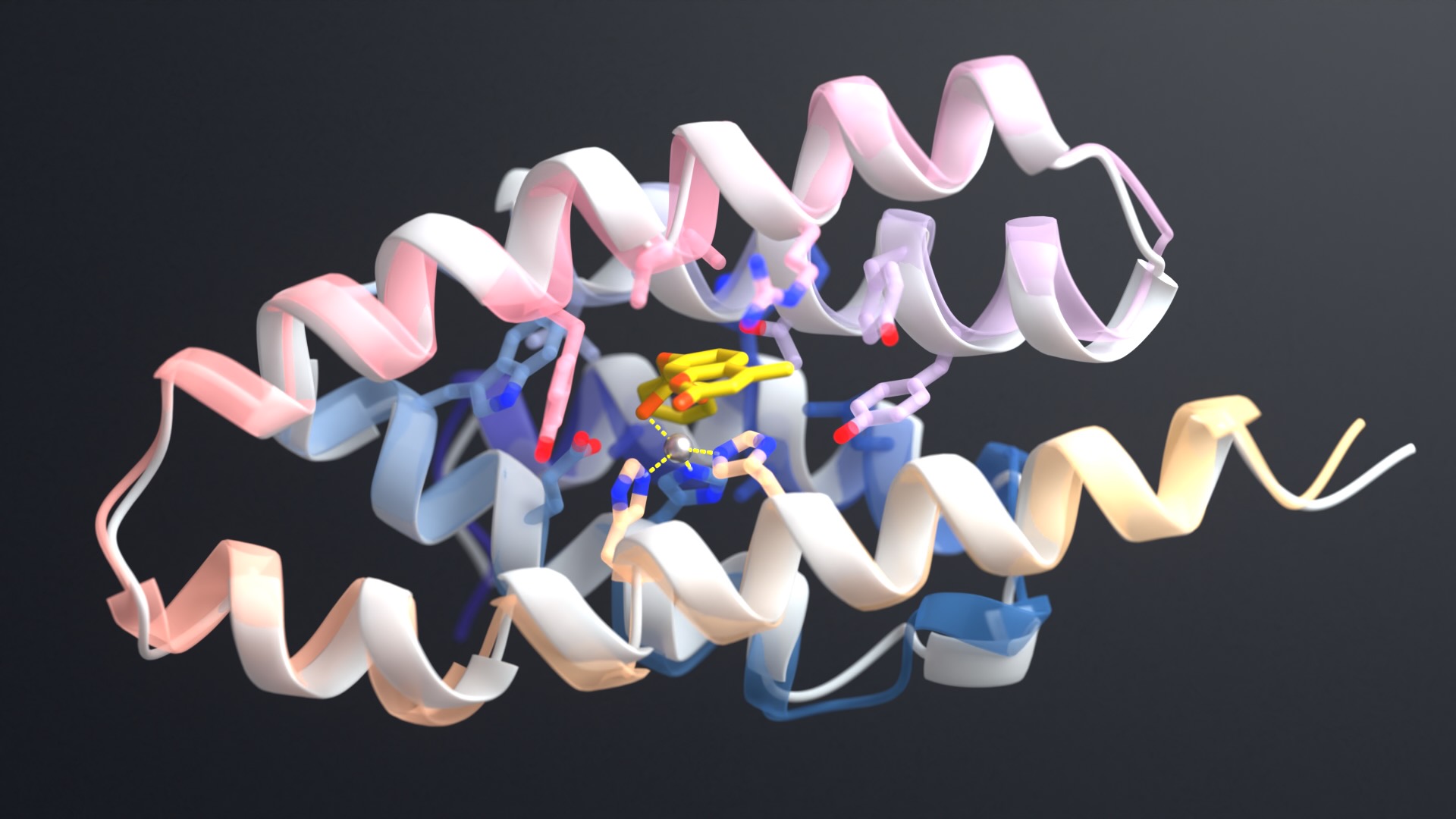
Today we are releasing RFdiffusion3 as open-source software. This state-of-the-art AI model for biodesign is capable of generating new proteins that interact with any type of molecule commonly found inside living cells.
“We built this as a general model and are sharing it freely so the scientific community can create things we haven’t even imagined yet.”
Lead developer Rohith Krishna, PhD
RFdiffusion3 tackles research challenges that have stymied protein engineers for decades. Building proteins that bind to specific DNA sequences — key for gene regulation and genome editing — has required researchers to specify the exact position of every atom in the molecules they wish to create. The same has been true for enzymes, which must position atoms with extreme precision to make and break chemical bonds.
Prior tools for molecular design attempted to simplify important chemical details, which limited what they could do. RFdiffusion3 takes a different approach.
“The central innovation here is precision control. We can now tell the model exactly which chemical interactions matter most, and it will generate new protein structures that make those interactions possible.”
Lead developer Jasper Butcher
This opens new possibilities for designing enzymes to break down microplastics, creating synthetic transcription factors for gene therapy, or building biosensors for environmental monitoring.
Key improvements
As detailed in a recent preprint, the team created RFdiffusion3 using the latest advances in efficient transformer architectures. The tool shares no code with its predecessors RFdiffusion or RFdiffusion2.
RFdiffusion3 delivers several key innovations:
- Efficiency and performance: RFdiffusion3 offers ten-fold faster performance over RFdiffusion2 developed earlier this year. In computational benchmarks, it matched or outperformed prior tools on a wide range of protein-protein binding, protein-DNA binding, protein-small molecule binding, and enzyme design tasks.
- Atom-level diffusion: The model treats individual atoms as the fundamental units being designed, applying a deep learning technique called diffusion to rapidly create new atomic arrangements. This produces intricate chemical interactions with unprecedented precision.
- A unified foundation model: RFdiffusion3 consolidates design capabilities that previously existed only in scattered, specialized models. Whether designing symmetric, binding, or catalytic proteins, researchers can now use a single, general-purpose tool.
Advancing open science
Training code and model weights for RFdiffusion3 are available on GitHub through the Rosetta Commons Foundry. Rosetta Commons is a collaborative network of labs that develops and maintains open-source software for biomolecular modeling.
The training code release is particularly significant. This allows scientists to adapt RFdiffusion3 to new problems, incorporate new data, and extend what the model can do — accelerating scientific progress in ways that closed tools cannot.
“When researchers from São Paulo to Nairobi can download the same code we use, that multiplies the rate of discovery. Improvements made by one lab benefit the entire community.”
IPD director David Baker, PhD
This work was funded by The Audacious Project, Microsoft, Howard Hughes Medical Institute, Open Philanthropy, National Institutes of Health, and other organizations. All funders are listed in the preprint, “De novo Design of All-atom Biomolecular Interactions with RFdiffusion3.”