Today we report in Cell our first computer-designed nanoparticle vaccine targeting respiratory syncytial virus, the primary cause of pneumonia in young children and the leading cause of infant mortality worldwide after malaria.
Although virtually every child will get infected by RSV before the age of three, an estimated 99 percent of deaths associated with the virus occur in developing countries. Despite substantial effort, there is not yet a safe and effective vaccine for RSV.
An international team co-led by researchers at our Institute has generated a first-of-its-kind vaccine that elicits broadly neutralizing antibodies against RSV in mice and monkeys, paving the way for human clinical trials.
“This is the first of many vaccine candidates we have made using this technology,” said senior author Neil King. By swapping out the proteins along the outside of the nanoparticle, Neil’s team hopes to create additional vaccines for diseases as diverse as HIV, malaria, and cancer.
Vaccines by design
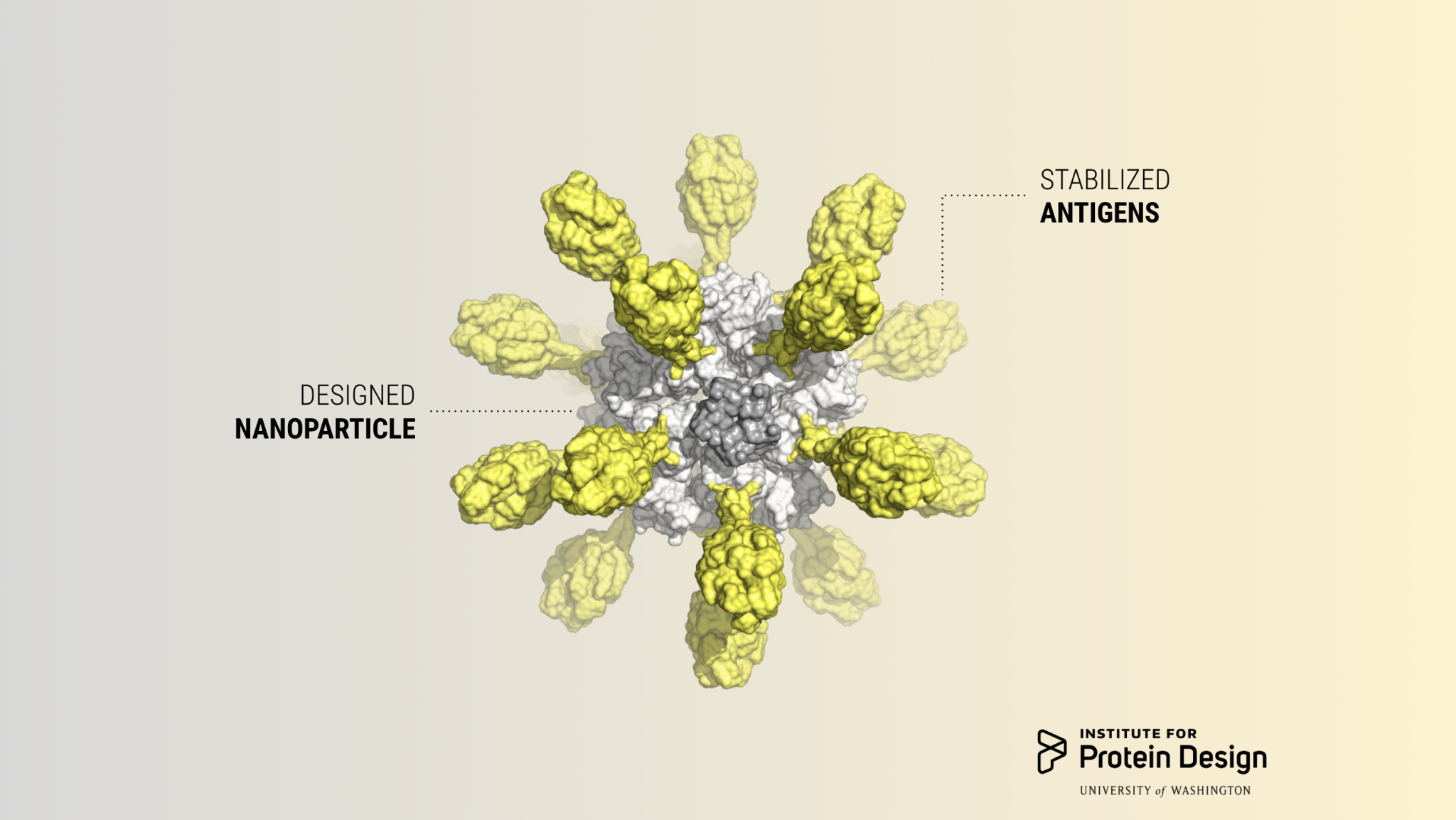
Scientists in the King Lab created the vaccine candidate by fusing DS-Cav1, a stabilized version of the viral glycoprotein RSV F which is responsible for membrane fusion, onto their designed two-component protein nanoparticle platform. This yielded a vaccine that can be tuned to display a variable number of antigens by mixing different versions of the purified parts.

Vaccine researchers Brooke Fiala and Neil King in the lab.
With a computer-generated protein nanoparticle at its core, the new multivalent vaccine candidate — dubbed DS-Cav1–I53-50 — is more stable than the trimeric antigen alone. In laboratory testing, it exhibited no discernible loss in antibody binding performance after being stored at elevated temperatures for two weeks. This may translate into a vaccine that does not require refrigeration, greatly reducing the cost and complexity of global vaccine distribution.
The new nanoparticle vaccine based on DS-Cav1 was also ten times more potent in initial tests than DS-Cav1 alone, suggesting it may translate into a more effective vaccine with more durable protection. DS-Cav1 was developed at the National Institute for Allergy and Infectious Disease Vaccine Research Center at the National Institutes of Health and has been shown to elicit significantly higher neutralizing antibody titers than postfusion F in animals and humans. DS-Cav1 is itself being evaluated in a Phase 1 study by NIH as an RSV vaccine candidate.
The RSV vaccine team was led by researchers at UW and the Institute for Research in Biomedicine in Bellinzona, Switzerland. It also included scientists from the Fred Hutch Cancer Research Center in Seattle, USA; the Karolinska Institute in Stockholm, Sweden; the Vaccine Formulation Institute in Godalming, UK; the European Virus Bioinformatics Center in Jena, Germany; the Vaccine Formulation Laboratory at the University of Lausanne, Switzerland; and the Institute of Microbiology at ETH Zürich, Switzerland. The project was funded in part by the Bill and Melinda Gates Foundation.
Read the full report here: https://www.cell.com/cell/fulltext/S0092-8674(19)30109-6 (PDF)