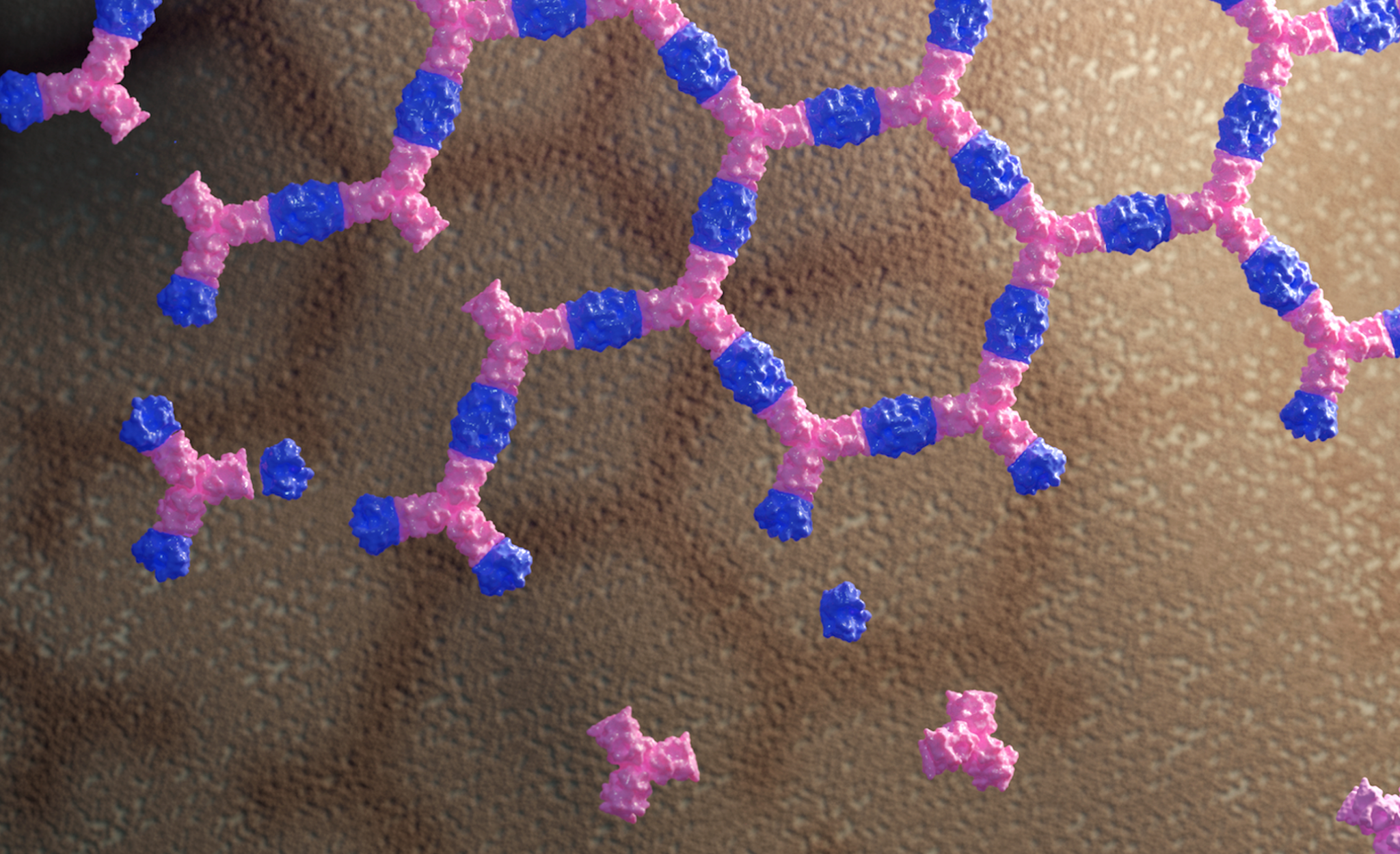
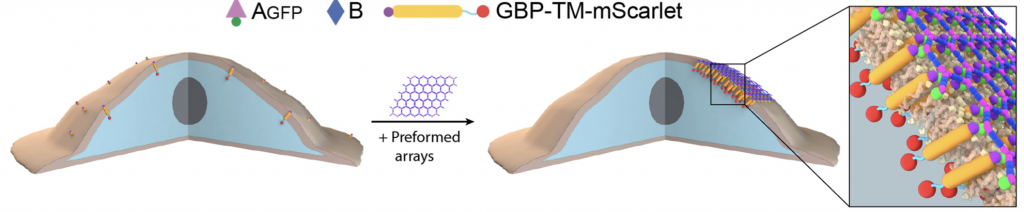
Today we report the design of a new class of protein material that interacts with living cells without being absorbed by them. These large, flat arrays built from multiple protein parts can influence cell signaling by clustering and anchoring cell surface receptors. This breakthrough could have far-reaching implications for stem cell research and enable the development of new materials designed to modulate the behavior of living systems.
The research, which appears in the January 6 edition of Nature [PDF], was led by the Baker lab at UW and the Derivery lab at the Medical Research Council Laboratory of Molecular Biology in Cambridge, UK.
Cells commonly terminate signaling by absorbing both an activated receptor and the molecule that stimulated it, targeting both for destruction inside the cell. “This tendency of cells to internalize receptors likely lowers the efficiency of immunotherapies,” said Emmanuel Derivery, assistant professor at the MRC Laboratory of Molecular Biology. “Indeed, when antibody drugs bind their target receptors and then become internalized and degraded, more antibody must always be injected.”
To create a way around this, Baker lab postdoctoral scholar Ariel Ben-Sasson designed new proteins that can assemble into large, flat patches upon an external cue. This molecular scaffolding was then further engineered to display signaling molecules. Graduate student Joseph Watson of the Derivery lab showed that such protein materials could latch onto cells, activate surface receptors, and resist being absorbed by the cell for hours or even days. By swapping out which cell surface receptors were targeted by the patch, the researchers showed that different cell types could be activated.
“We now have a tool that can interact with any type of cells in a very specific way. This is what is exciting about protein engineering — it opens fields that people may not expect.”

“This work paves the way towards a synthetic cell biology, where a new generation of multi-protein materials can be designed to control the complex behavior of cells,” said David Baker, professor of biochemistry and director of IPD.
According to co-author Hannele Ruohola-Baker, professor of biochemistry and associate director of the UW Institute for Stem Cell and Regenerative Medicine, versions of these new materials could eventually help physicians alleviate the dangers of sepsis by controlling the inflammatory response to infection and even enable entirely new ways to treat COVID-19, heart disease, and diabetes, and even mitigate the downstream effects of strokes, including Alzheimer’s disease. “This breakthrough helps pave the way for the use of synthetic cell biology in regenerative medicine,” said Ruohola-Baker.
This research was supported by the UK Medical Research Council, UK Engineering and Physical Sciences Research Council, Wellcome Trust, Human Frontier Science Program, Howard Hughes Medical Institute, US National Institutes of Health, US Department of Energy Office of Basic Energy Sciences Biomolecular Materials Program at Pacific Northwest National Laboratory, Medimmune, and Infinitus.