Two different candidate vaccines developed by researchers at the Institute for Protein Design recently entered human clinical trials. GBP510, a candidate COVID-19 vaccine, is undergoing a combined Phase 1/2 trial. Flu-Mos-v1, a candidate mosaic influenza vaccine, is undergoing Phase 1 testing.
Candidate COVID-19 vaccine
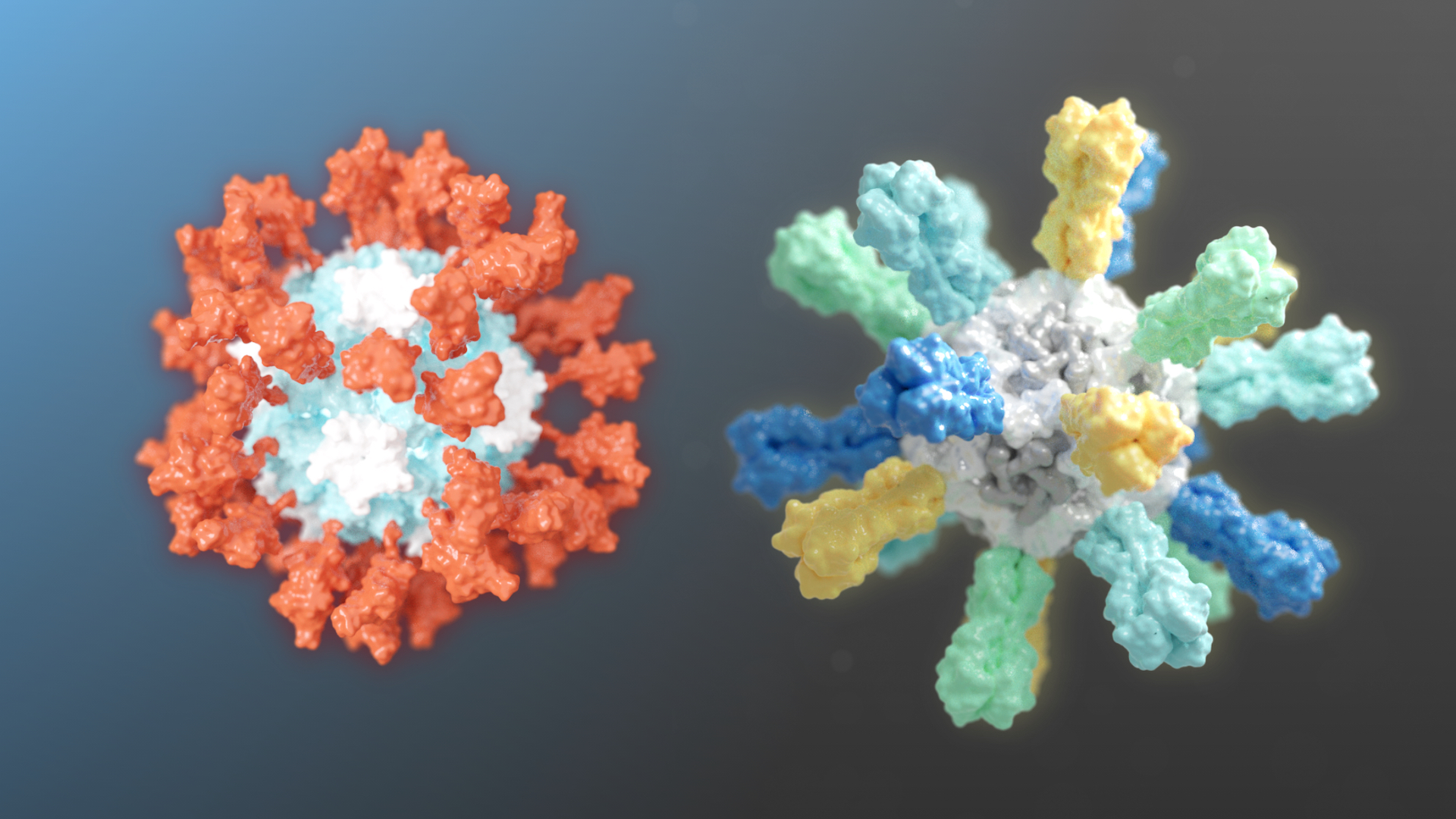
Our SARS-CoV-2 vaccine candidate was created with structure-based vaccine design techniques invented in the King lab the IPD. It is based on a computationally designed self-assembling protein nanoparticle that displays 60 copies of a key region of the viral Spike protein.
In preclinical studies reported last year in Cell, the vaccine produced high levels of virus-neutralizing antibodies at low doses. Compared to vaccination with the soluble SARS-CoV-2 Spike protein, on which many leading COVID-19 vaccine candidates are based, the nanoparticle vaccine produced 10 times more neutralizing antibodies in mice, even at a sixfold lower dose. When administered to a single nonhuman primate, it produced neutralizing antibodies targeting multiple different sites on the Spike protein. This may ensure protection against additional mutated strains of the virus, should they arise.
Further preclinical studies, published in Nature, also showed that the vaccine conferred robust protection and produced a strong B-cell response, which may improve how long the protective effects of the vaccine last.
Our lead COVID-19 nanoparticle vaccine candidate is being licensed non-exclusively and royalty-free during the pandemic by the University of Washington. One licensee, Icosavax, a Seattle biotechnology company co-founded in 2019 by King, is currently advancing studies to support regulatory filings and has initiated the U.S. Food and Drug Administration’s Good Manufacturing Practice. To accelerate progress by Icosavax to the clinic, Amgen has agreed to manufacture a key intermediate for these initial clinical studies.
Another licensee, SK bioscience of South Korea, is advancing its own studies to support clinical and further development. Lab studies conducted at SK bioscience offered additional evidence that the nanoparticle vaccine blocked proliferation of the COVID-19 virus.
SK bioscience is working toward commercialization within the first half of 2022 through an expedited approval process, such as an emergency use license. Their eventual goal would be to build up to a manufacturing scale of hundreds of millions of doses per year. GBP510, as SK has named the program, was selected for the first program of the Wave 2 (Next Generation COVID-19 Vaccine) project, which CEPI launched last year to support various COVID-19 vaccine candidates. If GBP510 proves safe and effective and becomes commercialized, it will be supplied globally through the COVAX Facility.
In addition to Neil King, head of vaccine design at the IPD and inventor of the computational design technology used in developing this COVID-19 nanoparticle vaccine candidate, other lead investigators are research scientists Alexandra Walls and Brooke Fiala, and David Veesler, associate professor, all in the University of Washington School of Medicine Department of Biochemistry, who conducted the work along with numerous collaborators.
Mosaic influenza vaccines
IPD researchers, together with collaborators at the National Institutes of Health, have developed experimental flu vaccines that protect animals from a wide variety of seasonal and pandemic influenza strains. The vaccine recently entered Phase 1 clinical testing. If proven safe and effective, these next-generation influenza vaccines may replace current seasonal options by providing protection against many more strains that current vaccines do not adequately cover.
A study detailing how the new flu vaccines were designed and how they protect mice, ferrets, and nonhuman primates was published in Nature. This work was led by researchers at the University of Washington School of Medicine and the Vaccine Research Center part of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
“Most flu shots available today are quadrivalent, meaning they are made from four different flu strains. Each year, the World Health Organization makes a bet on which four strains will be most prevalent, but those predictions can be more or less accurate. This is why we often end up with ‘mismatched’ flu shots that are still helpful but only partially effective,” said lead author Daniel Ellis, a research scientist in the King lab.
Dr. Anthony Fauci shared some news with Congress about our collaborative influenza vaccine research. Phase 1 trials are now underway!
Paper is https://t.co/RWqnA9d7kG by @DanLikeProteins, @KingLabIPD, @kanekiyom, @BarneyGrahamMD, & many more. pic.twitter.com/lTEYaletYI
— Institute for Protein Design (@UWproteindesign) May 28, 2021
To create improved influenza vaccines, the team attached hemagglutinin proteins from four different influenza viruses to custom protein nanoparticles. This approach enabled an unprecedented level of control over the molecular configuration of the resulting vaccine and yielded an improved immune response compared to conventional flu shots. The new nanoparticle vaccines, which contain the same four hemagglutinin proteins of commercially available quadrivalent influenza vaccines, elicited neutralizing antibody responses to vaccine-matched strains that were equivalent or superior to the commercial vaccines in mice, ferrets, and nonhuman primates. The nanoparticle vaccines—but not the commercial vaccines —also induced protective antibody responses to viruses not contained in the vaccine formulation. These include avian influenza viruses H5N1 and H7N9, which are considered pandemic threats.
“The responses that our vaccine gives against strain-matched viruses are really strong, and the additional coverage we saw against mismatched strains could lower the risk of a bad flu season,” said Ellis.
Initial clinical testing of the leading nanoparticle influenza vaccines is expected to take up to two years.