Category: Coronavirus
-

Annual Report 2022
Protein design reached two major milestones this year: Our Institute succeeded in producing its first fully-approved medicine, and our spinout companies have together raised over one billion dollars in capital. We are pleased to present this overview of the progress made at the Institute for Protein Design during the past year. 2022 Annual Report
-

COVID-19 vaccine with IPD nanoparticles wins full approval abroad
Clinical testing found the vaccine outperforms Oxford/AstraZeneca’s. The vaccine, now called SKYCovione, is the world’s first computationally designed protein medicine. University of Washington to waive royalty fees for the duration of the pandemic. A vaccine for COVID-19 developed at the University of Washington School of Medicine has been approved by the Korean Ministry of Food…
-

AWS gift supports protein structure prediction and design
Amazon Web Services (AWS), a leading cloud computing platform, is donating server time to the Institute for Protein Design to accelerate research in protein structure prediction and design. Computing credits valued at over $1M will be used to train optimized versions of RoseTTAFold for higher accuracy. The research will also support the ongoing development of…
-
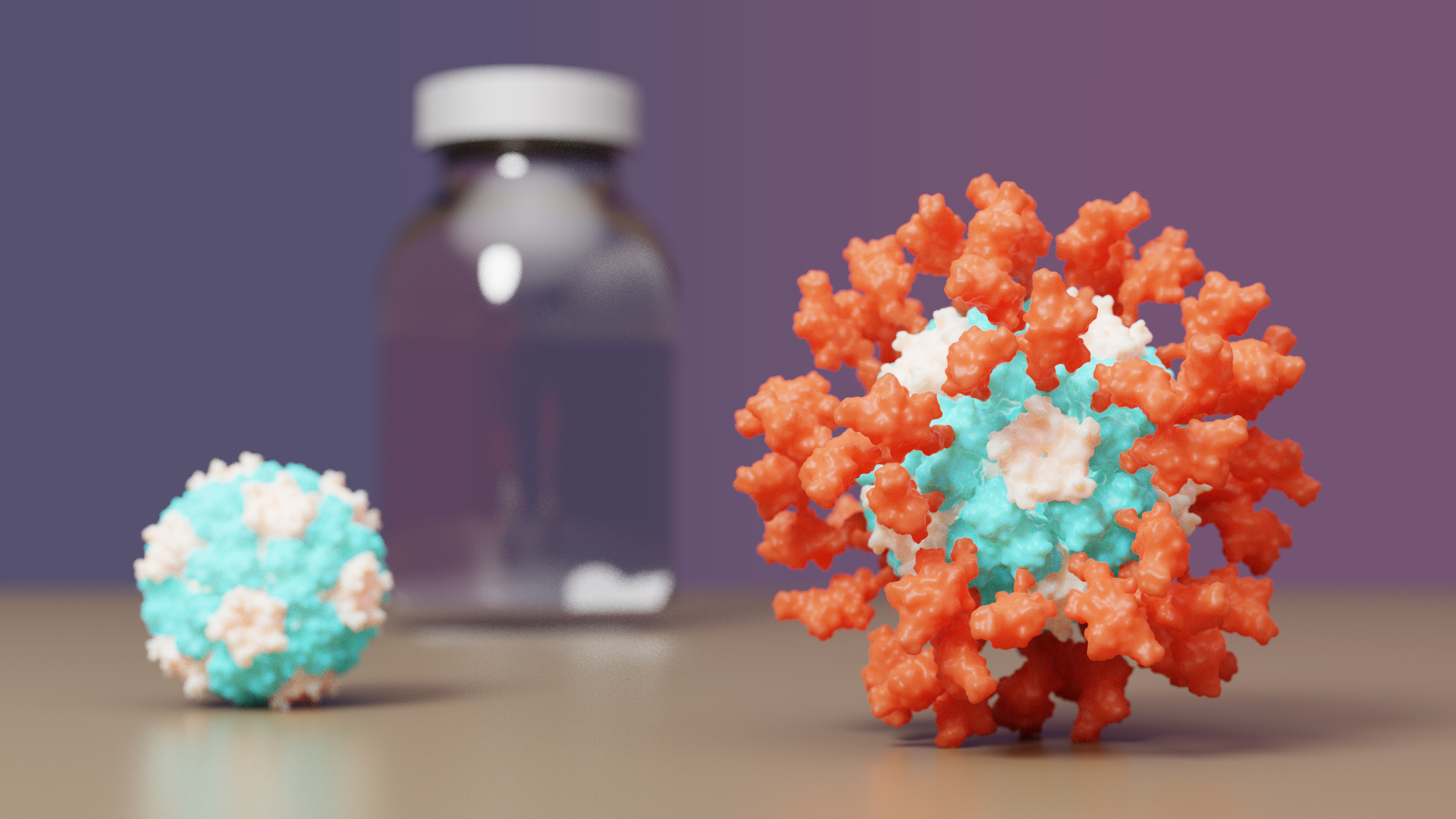
COVID-19 vaccine with IPD nanoparticles seeks full approval
A COVID-19 vaccine developed at the University of Washington School of Medicine has proven safe and effective in late-stage clinical testing. SK bioscience, the company leading the vaccine’s clinical development abroad, is seeking full approval for its use in South Korea and beyond. If approved by regulators, the vaccine will be made available through COVAX,…
-
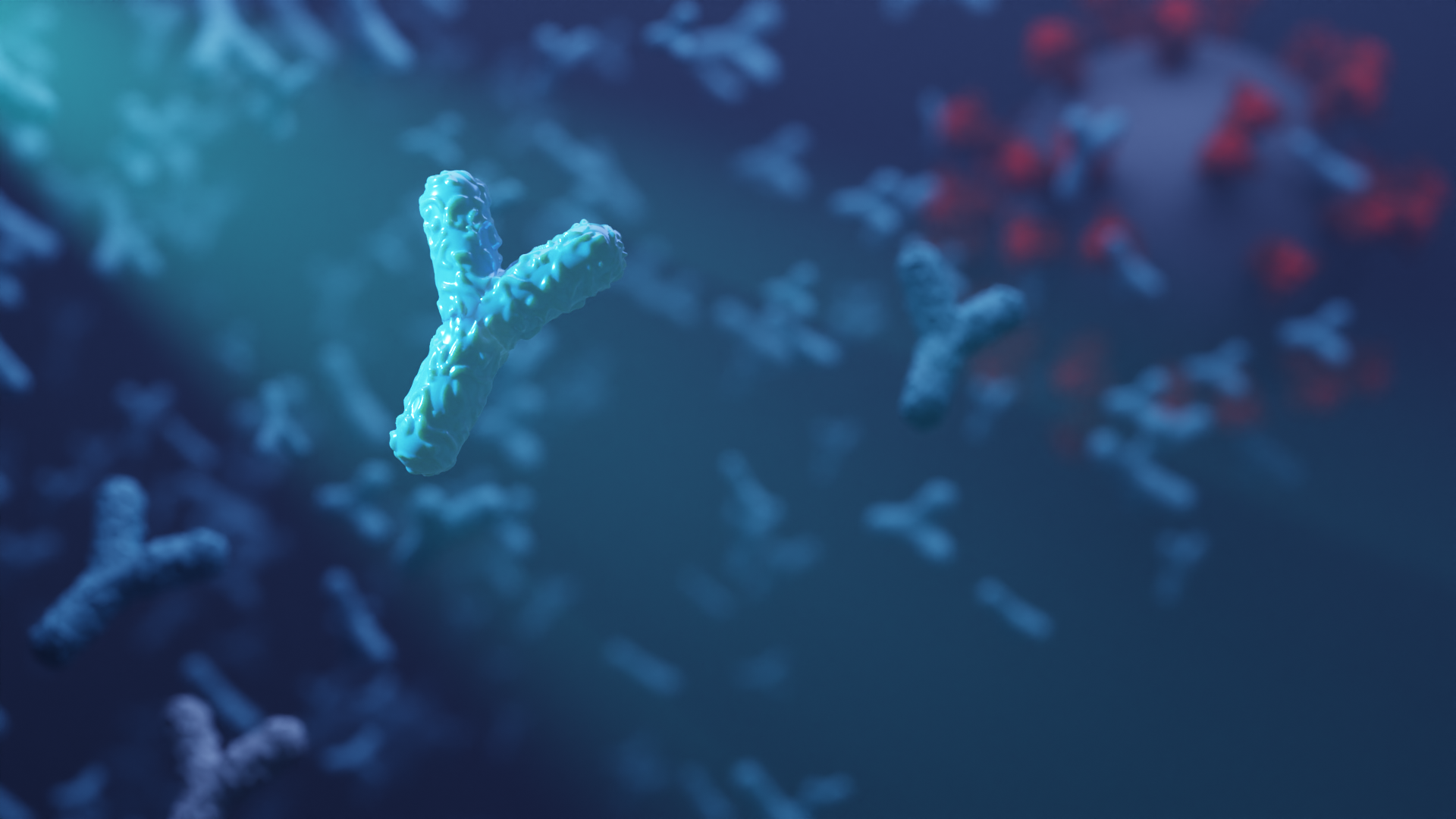
Custom biosensors for detecting coronavirus antibodies in blood
Today we report in Nature Biotechnology the design of custom protein-based biosensors that can detect coronavirus-neutralizing antibodies in blood. This research, which builds on prior sensor design technology developed in the Baker lab, was led by Baker lab postdoctoral scholars Jason Zhang, PhD, and Hsien-Wei (Andy) Yeh, PhD. From Behind the Paper: [W]e utilized the…
-
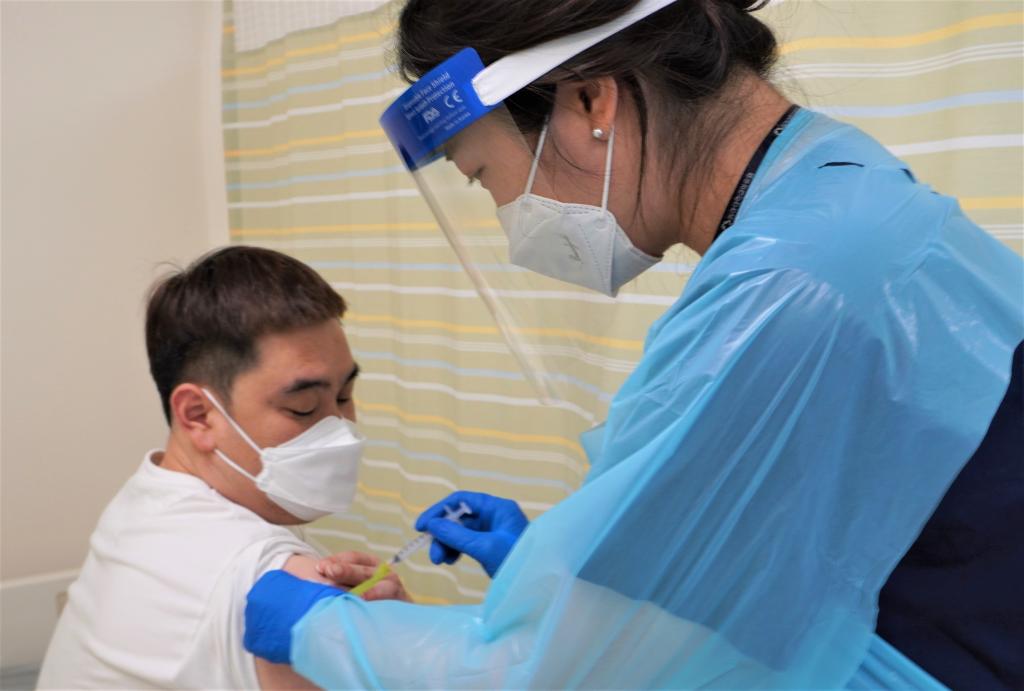
COVID-19 vaccine with IPD nanoparticles meets Phase 1/2 trial goals
This report was written and translated into English by SK bioscience. (Image: SK bioscience) SK bioscience (CEO Jae-yong Ahn) announced on November 4th that the company has confirmed a positive immune response and safety in the final analysis result of the phase I/II clinical trial of the COVID-19 vaccine candidate, ‘GBP510,’ co-developed with the Institute…
-

Baker lab joins USAID’s $125M project to detect emerging viruses
To better identify and prevent future pandemics, the University of Washington has become a partner in a five-year global, collaborative agreement with the U.S. Agency for International Development. The newly launched Discovery & Exploration of Emerging Pathogens – Viral Zoonoses, or DEEP VZN project, has approximately $125 million in anticipated funding and will be led…
-

Annual Report 2021
From our deep-learning research to our new clinical trials, we are pleased to present this overview of the progress made at the Institute for Protein Design during the past year. 2021 Annual Report
-
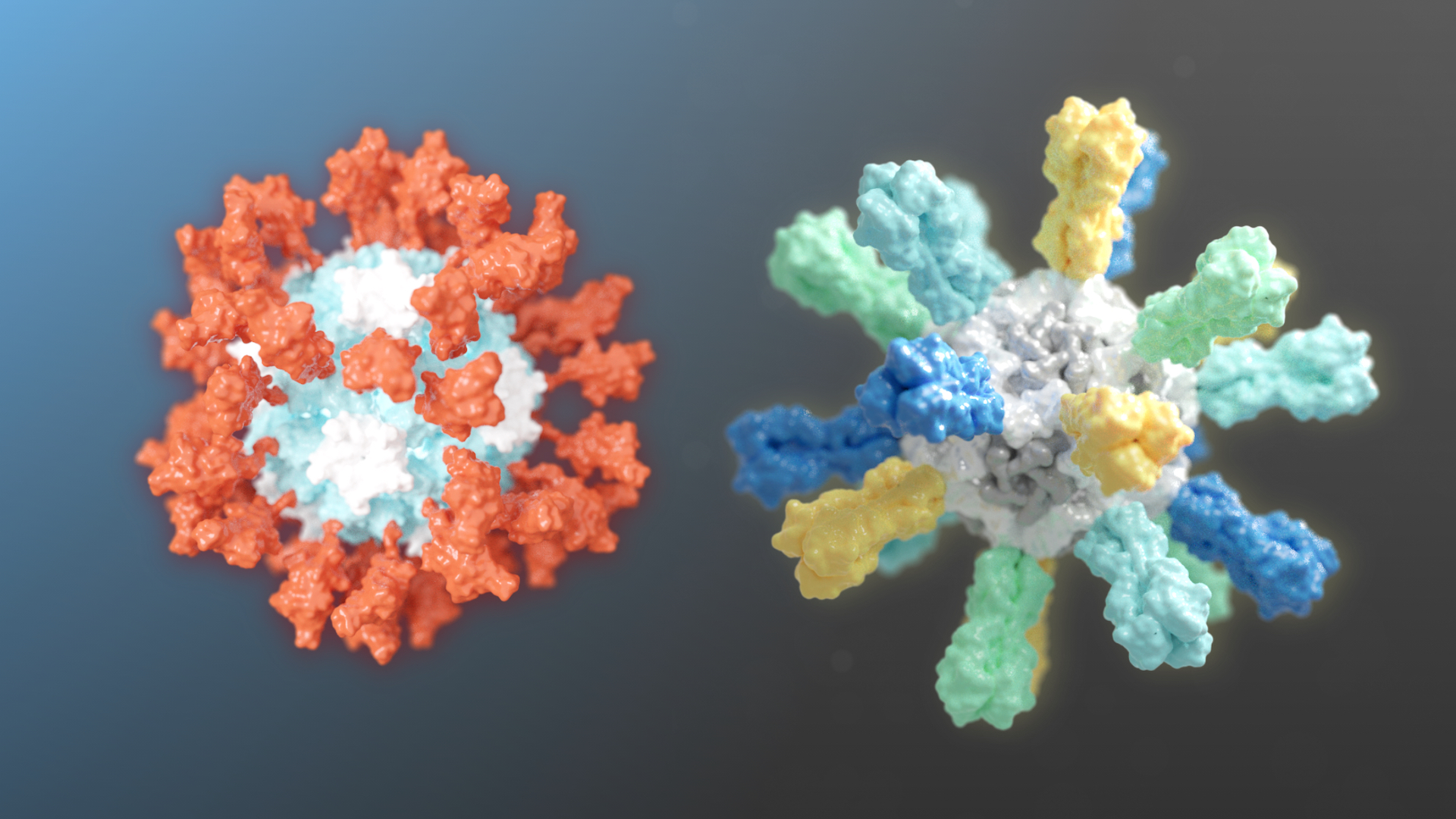
Two nanoparticle vaccines enter clinical trials
Two different candidate vaccines developed by researchers at the Institute for Protein Design recently entered human clinical trials. GBP510, a candidate COVID-19 vaccine, is undergoing a combined Phase 1/2 trial. Flu-Mos-v1, a candidate mosaic influenza vaccine, is undergoing Phase 1 testing. Candidate COVID-19 vaccine Our SARS-CoV-2 vaccine candidate was created with structure-based vaccine design techniques…
-
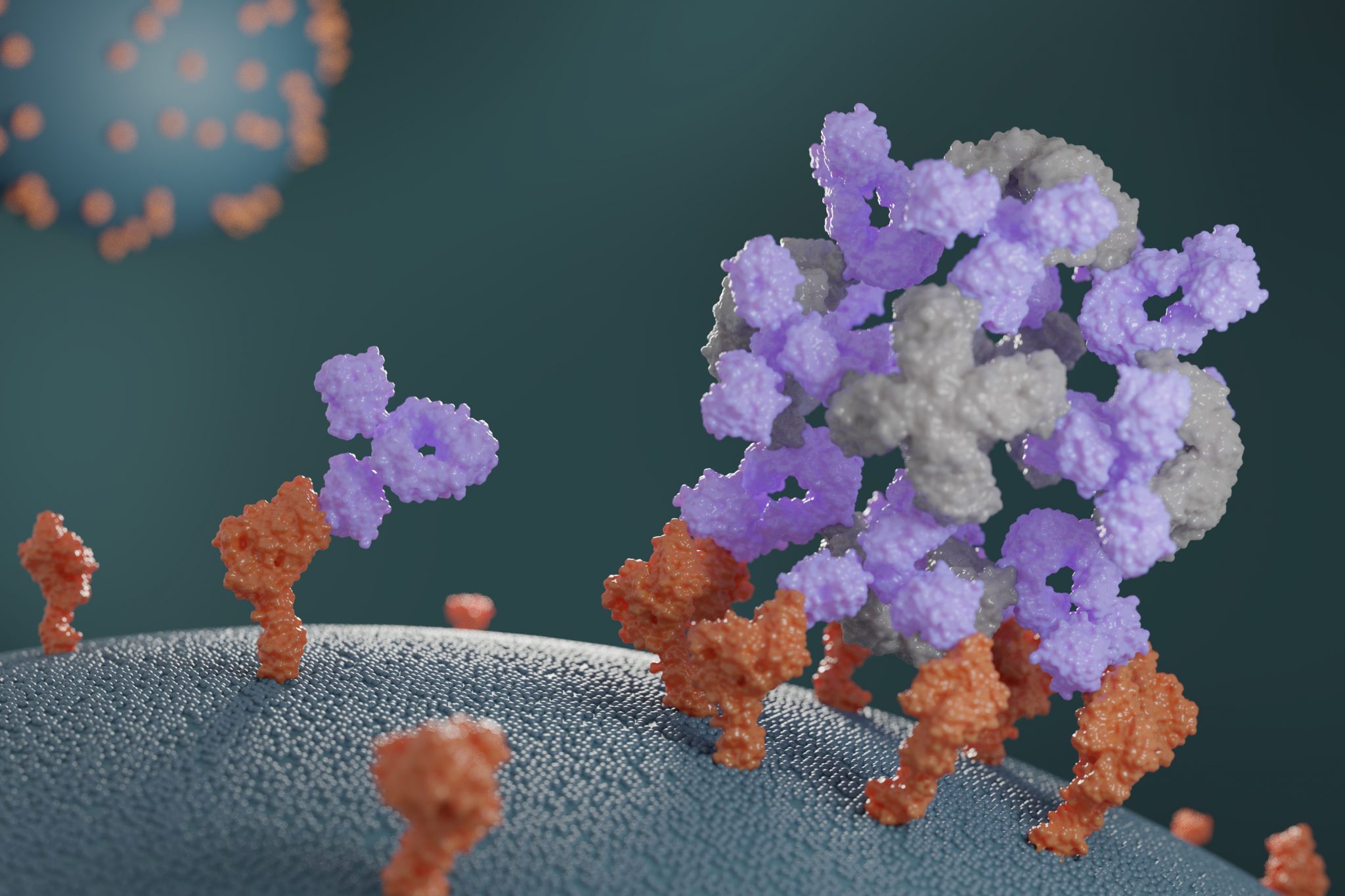
Companion proteins enhance antibody potency
This week we report the design of new proteins that cluster antibodies into dense particles, rendering them more effective. In laboratory testing, such clustered antibodies neutralize COVID-19 pseudovirus, enhance cell signaling, and promote the growth of T cells more effectively than do free antibodies. This new method for enhancing antibody potency may eventually be used…
-
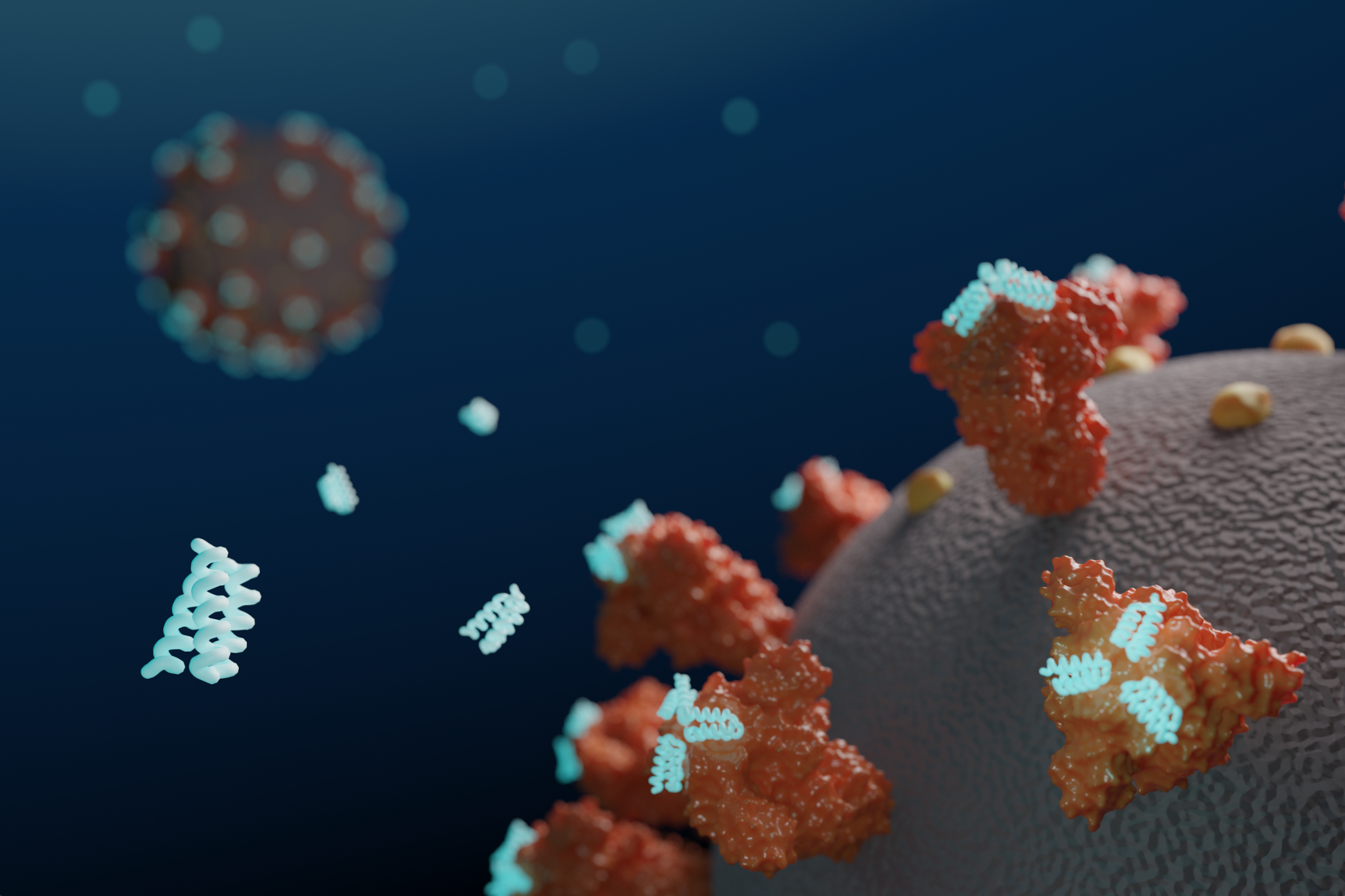
New sensors detect coronavirus proteins and antibodies
This week we report [PDF] a new way to detect the virus that causes COVID-19, as well as antibodies against it. Scientists at the Institute for Protein Design have created protein-based sensors that glow when mixed with components of the virus or specific antibodies. This breakthrough could enable faster and more widespread testing in the near…
-
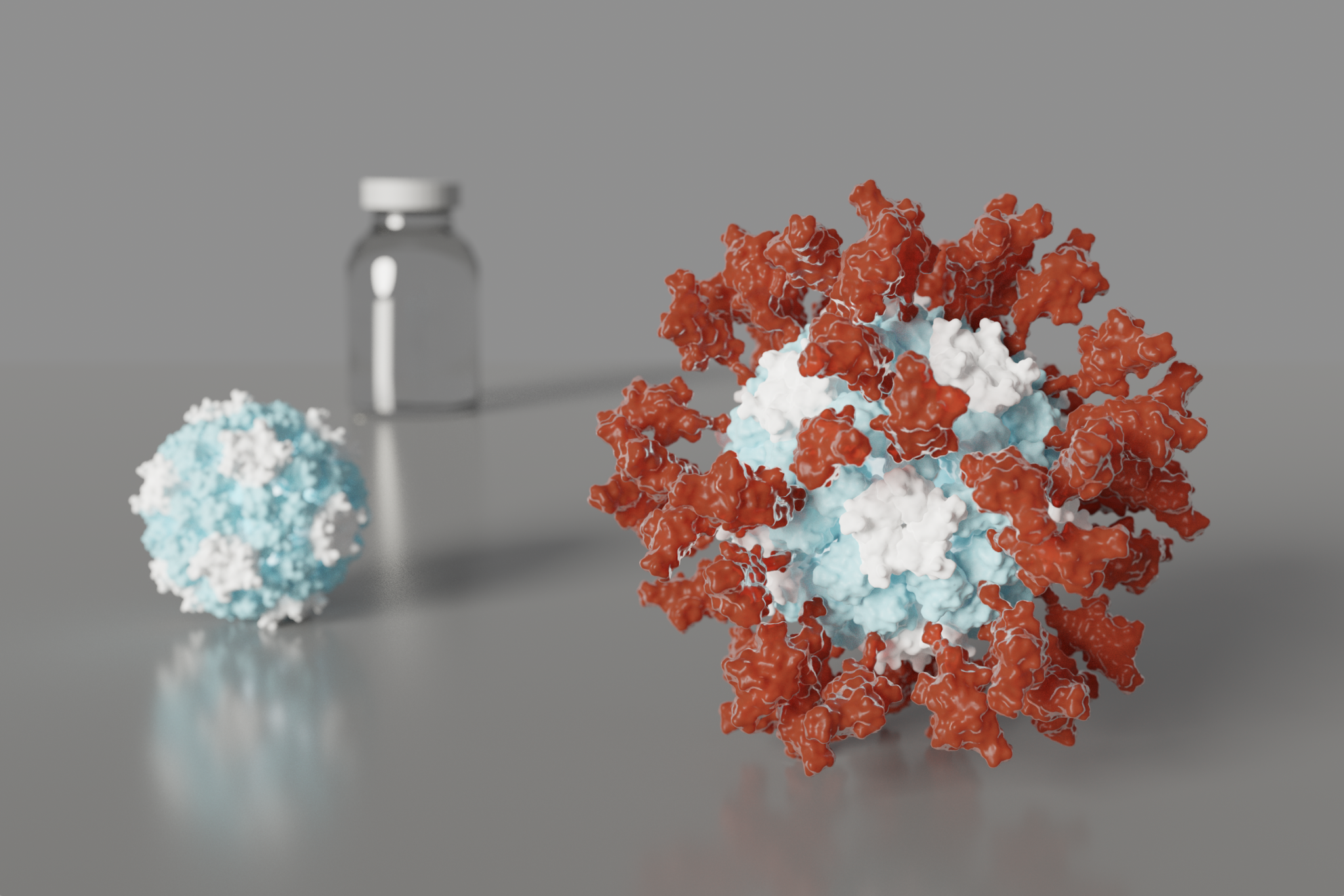
Design of an ultrapotent COVID-19 vaccine candidate
Today we report in Cell (PDF) the design and initial preclinical testing of an innovative nanoparticle vaccine candidate for the pandemic coronavirus. It produces virus-neutralizing antibodies in mice at levels ten-times greater than is seen in people who have recovered from COVID-19. Compared to vaccination with the soluble SARS-CoV-2 Spike protein, which is what many leading…
-
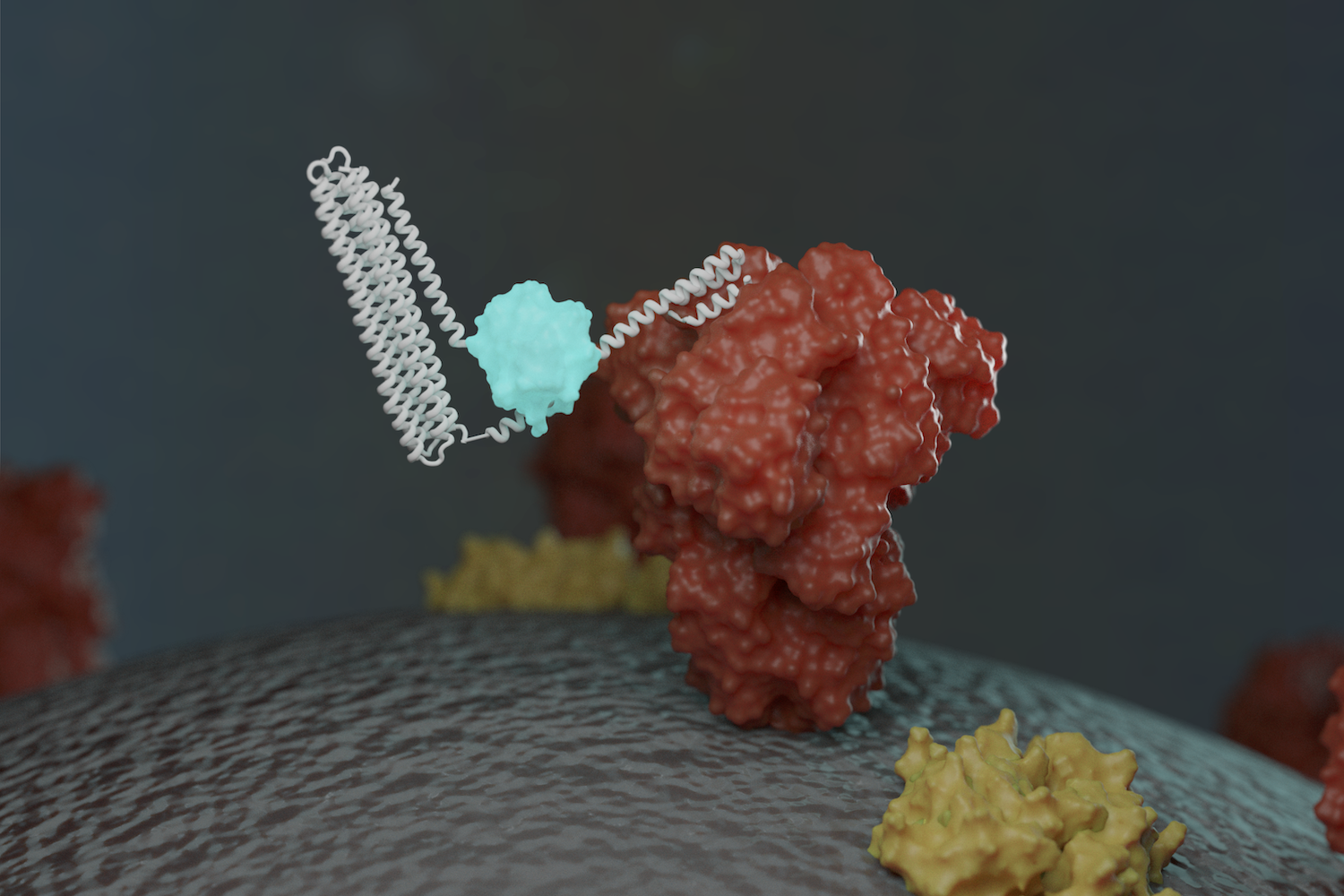
Antiviral proteins block coronavirus infection in the lab
Today we report in Science [PDF] the design of small proteins that protect cells from SARS-CoV-2, the virus that causes COVID-19. In experiments involving lab-grown human cells, the activity of the lead antiviral candidate produced (LCB1) was found to rival that of the best-known SARS-CoV-2 neutralizing antibodies. LCB1 is currently being evaluated in rodents. Coronaviruses…
-

Annual Report 2020
From our COVID-19 response to spinout highlights, we are pleased to present this overview of the progress made at the Institute for Protein Design during the past year. Annual Report 2020
-
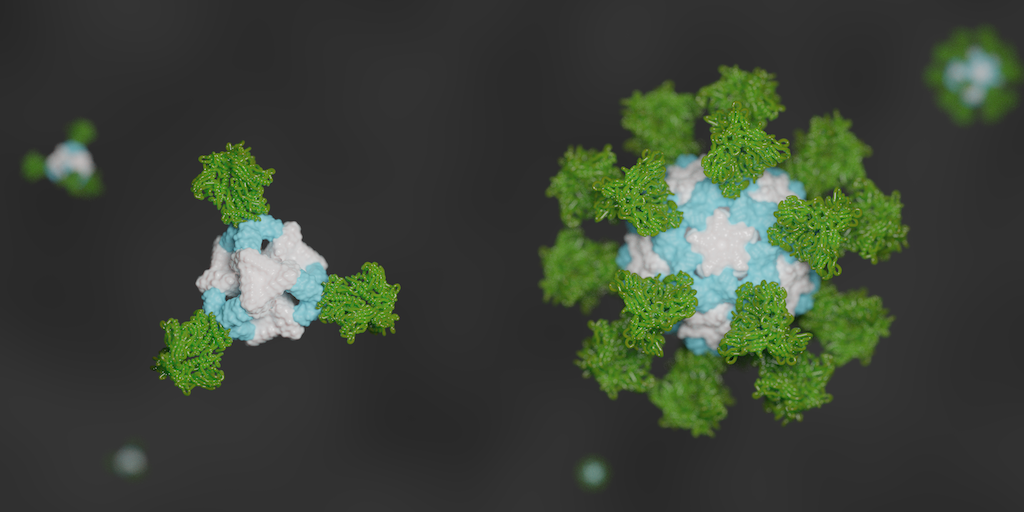
De novo nanoparticles as vaccine scaffolds
IPD researchers have developed a new vaccine design strategy that could confer improved immunity against certain viruses, including those that cause AIDS, the flu, and COVID-19. Using this technique, viral antigens are attached to the surface of self-assembling, de novo designed protein nanoparticles. This enables an unprecedented level of control over the molecular configuration of the resulting…
-
With an emergency meeting, RosettaCommons aims to accelerate COVID-19 research
On the day the number of confirmed global COVID-19 infections crossed one million, a team of over three hundred scientists from around the world kicked off a virtual conference aimed at stopping the virus. The event was an emergency meeting of member labs from the RosettaCommons, a consortium of over 90 laboratories who together develop…
-

5 questions about designing coronavirus vaccines from our Reddit AMA
Researchers from our vaccine design team recently participated in a Reddit ‘Ask Me Anything’ about our SARS-CoV-2 vaccine research. Reddit users asked over a hundred questions by the time the live event ended — we are sorry we could not address them all. We were lucky to be joined by Lexi Walls, a postdoctoral scholar…
-

Volunteers rally to Rosetta@Home to stop COVID-19
As schools, museums, offices and stores shutter to slow the spread of the new coronavirus, millions of people are now finding themselves stuck at home. Fortunately, even in these trying times, there are are small steps that anyone can be take to help combat COVID-19. One option is to donate to biomedical research — but…
-

Play Foldit to help stop coronavirus (VIDEO)
You don’t have to be a scientist to do science! By playing the computer game Foldit, you can help discover new antiviral drugs that might stop the novel coronavirus. The most promising solutions will be manufactured and tested at the University of Washington Institute for Protein Design in Seattle. Foldit is run by academic research scientists. It is free…
-
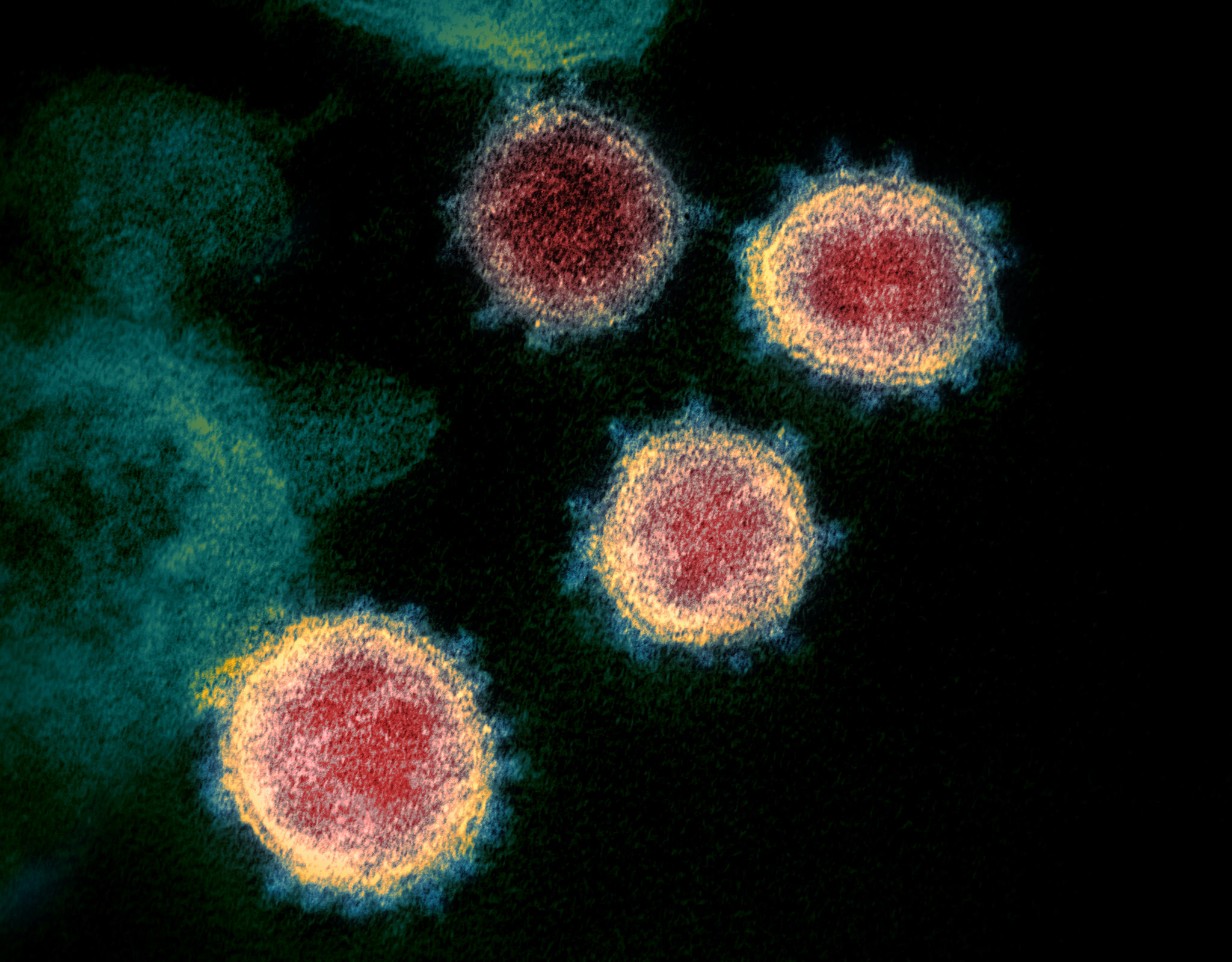
Rosetta’s role in fighting coronavirus
We are happy to report that the Rosetta molecular modeling suite was recently used to accurately predict the atomic-scale structure of an important coronavirus protein weeks before it could be measured in the lab. Knowledge gained from studying this viral protein is now being used to guide the design of novel vaccines and antiviral drugs.…
