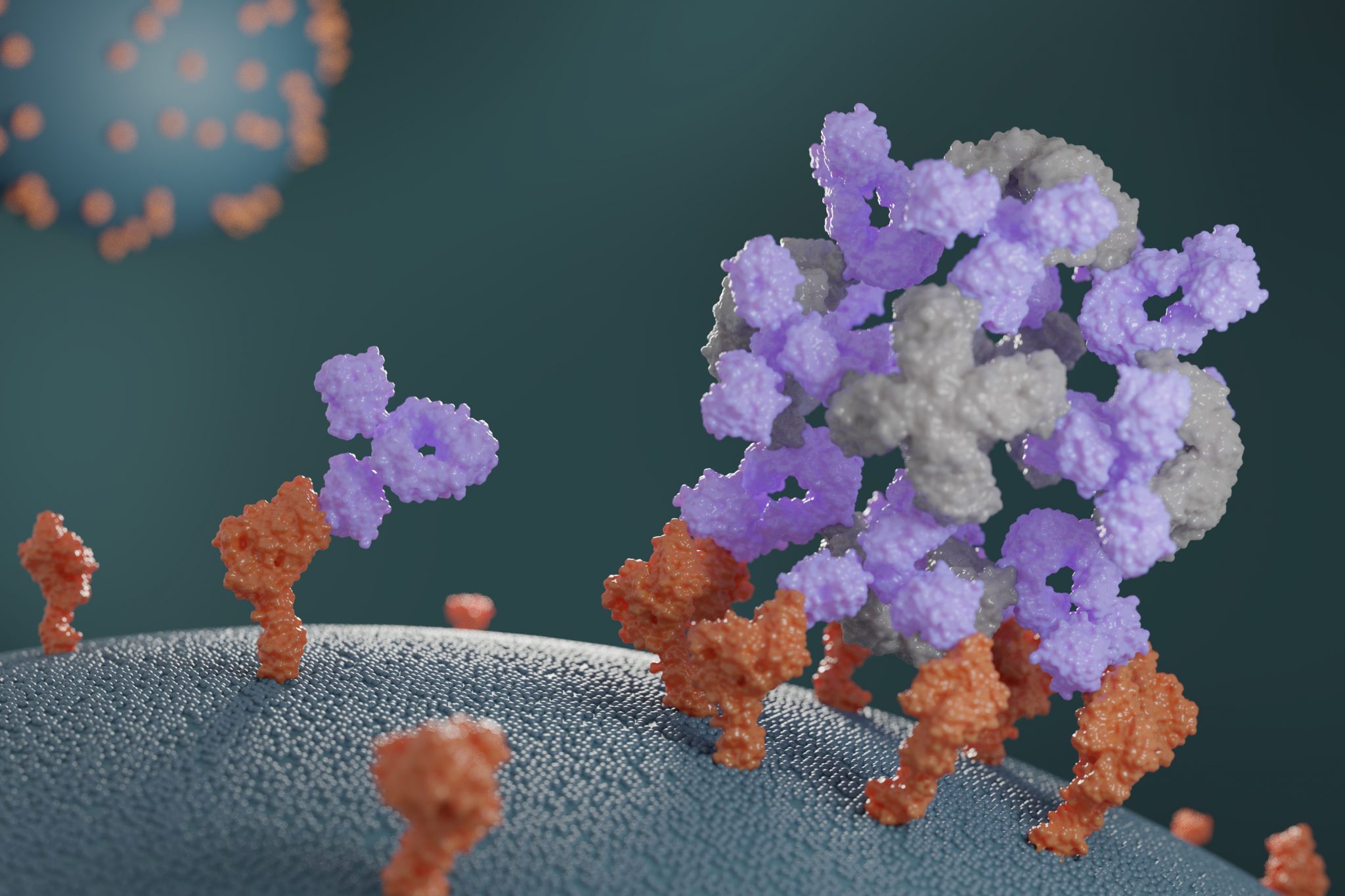
This week we report the design of new proteins that cluster antibodies into dense particles, rendering them more effective. In laboratory testing, such clustered antibodies neutralize COVID-19 pseudovirus, enhance cell signaling, and promote the growth of T cells more effectively than do free antibodies. This new method for enhancing antibody potency may eventually be used to improve antibody-based treatments for a wide range of health disorders.
Antibodies are essential tools in modern medicine, accounting for more than half of all best-selling drugs in recent years. They are used to treat arthritis, cancer, autoimmune disorders, COVID-19, and much more. In 2019, the market for antibody-based technologies reached $150 billion.
Antibodies are typically free-floating proteins. They function by binding to a specific molecular target, which then becomes either activated or inactivated. Virus-neutralizing antibodies that adhere to the surface of the coronavirus can render the pathogen inert. Signaling antibodies that latch onto human cell receptors can alter cellular communication, metabolism, and even gene expression in profound ways.
“We knew that clustering other kinds of signaling proteins can greatly enhance their effects, but there have not been good ways of clustering antibodies,” said lead-author Robby Divine, a graduate student in the department of biochemistry at UW Medicine. Divine led a team of scientists that used molecular design software to create proteins that recognize and bind to specific surfaces that are common to all human antibodies — the so-called fragment crystallizable, or Fc, region.
“Initially, we were just curious to see if we could build proteins that would grab hold of antibodies,” said Divine. With continued bioengineering, the team eventually created proteins that not only bound to antibodies but also assembled them into dense, spherical nanoparticle structures. They call these structures ‘antibody nanocages.’
“Some of the first cages we made would only grab two antibodies per cluster, but we later created cages that could bind six, 12, or even 30. And we quickly found that any antibody we tested could pretty easily be put into a nanocage. It was surprising to see how generic this approach was.”

So far, antibody nanocages have yielded promising results in various laboratory tests. Certain antibodies known to neutralize the COVID-19 coronavirus become seven-fold more potent when formulated into a nanocage. Other antibodies that induce signaling of a protein called CD40 in mammalian cells functioned around 20-fold more potently when formulated into a nanocage, allowing for 20-fold lower dosing to achieve identical signaling results. And when the once-promising anti-cancer antibody conatumumab was formulated into a nanocage, it was able to potently trigger cell death in lab-grown cancer cells. The same antibody alone did not promote cancer cell death, even at the highest concentrations tested. More experiments will be needed to establish whether these trends hold in animal testing.
“The most exciting aspect of this technology is that it is so simple to swap different antibodies into the assemblies. We envision many new treatments emerging from this one common tool,” said senior author David Baker, professor of biochemistry and director of the UW Medicine Institute for Protein Design.
This work was led by UW Medicine and included researchers from the Benaroya Research Institute and Fred Hutchinson Cancer Research Center, Seattle, WA, USA; and Tehran University of Medical Sciences, Tehran, Iran. This work was supported by the National Institutes of Health, National Science Foundation, Howard Hughes Medical Institute, Washington Research Foundation, Audacious Project, Nordstrom-Barrier Directors Fund, Washington State General Operating Fund, Wu Tsai Translational Investigator Fund, Nan Fung Life Sciences Translational Investigator Fund, Fred Hutch COVID-19 Research Fund, a Pew Biomedical Scholars Award, and a Burroughs Wellcome Investigators award.